
With this experience, you will understand that fire needs oxygen and that in the absence of it, the candle goes out. The trick is therefore to eliminate the oxygen around the candle. Carbon dioxide is an inert gas used in fire extinguishers. When it replaces the air around the candle … the fire goes out. Try to make your own fire extinguisher.
You will need:
- A candle
- A small bottle of soda
- 2 t. s. of sodium bicarbonate
- Vinegar
- Matches

From 6 years

Difficulty : easy
This experience requires the help of an adult

Let's experiment


Gather the required materials


In the plastic or glass bottle, add the baking soda.

Add a t. s. of vinegar and observe!

You immediately observe the appearance of bubbles. It means that a reaction is occurring in your bottle.


Now approach the neck of the bottle near the flame of the candle.


You observe that the gas released by the bottle extinguishes the flame of the candle!
Understand the experiment

Don't pour fuel on the fire!
Baking soda is what we call a “base”, while vinegar is named an “acid”. When these two products come into contact, a reaction is occuring, with the immediate release of gas. It is called an “acid-base” reaction.
Similar experiences
Why does the fire go out then?
To keep burning, fire needs three elements : heat, fuel and an oxidizing agent (oxygen). Oxygen is a gas present in the atmosphere. As it maintains the burning of the candle, scientists call it “oxidizer” or “oxidizing agent”
The gas released during our reaction is carbon dioxide. It is denser than oxygen in the air. When leaving the bottle, it deprives the flame of the presence of this essential element. It is, therefore, expected that the candle goes out due to a lack of oxygen.

Did you know?
The little story of extinguisher
In the 19th century, a baking soda-based fire extinguisher was created. Instead of vinegar, scientists used sulfuric acid, an extremely powerful and corrosive acid. The sudden release of the gas allowed the rapid expulsion of the water contained in the tank (6L) to extinguish the fire.
In the mid-20th century, the potential of carbon dioxide was more widely exploited with new fire extinguishers releasing carbon dioxide under pressure. The rapid expansion of gas allows filling the room with inert, non-combustible gas.
Challenge
Place several candles in a line. Light them and see how many candles you can put out at once.
Tips: you can also direct the carbon dioxide out of your bottle using a straw, firmly attached to the cap of your bottle with tape.
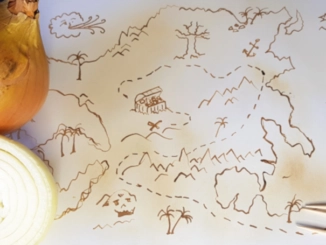
A treasure map with invisible ink
Invisible ink is very useful for drawing treasure maps or writing secret messages. Only those who know chemistry will find a way to reveal your message. Let’s use onion juice to prepare the invisible ink. […]

Prepare an orange caviar
Use molecular chemistry to create foods with unexpected shapes. Do you think making orange juice beads is impossible? This is without counting on the properties of Agar, a natural molecule capable of gelling all liquids. Transform yourself into a molecular chemist to surprise your friends with your edible creations. […]

The volcano science experiment
Observing a chemical reaction in your kitchen is possible with the volcano science experiment. Build your volcano with sand. And then let’s move on to making lava. […]




