
We will recreate 1001 colors with red cabbage. How do we do it? Let’s discover it together.
Have you ever noticed how lemon juice or vinegar stings the tongue? They are said to be acidic. That’s a funny word. In nature, some compounds are acidic, and others are basic, like bleach. Some are so strong that they are dangerous to humans. Like in the cartoons, these products can burn your skin! But how do you find your way around all these products? To help you out, take a red cabbage! You’ll see how interesting this vegetable is for identifying everyday products. Shall we give it a try?
You will need:
- A fresh or jarred red cabbage
- A knife
- A pan filled with water
- 7-8 coffee filters
- A paintbrush
- Various liquids: soda, vinegar, detergent, water, bleach

From 8 years old

medium
This experience requires the help of an adult

Let's experiment


With the help of an adult, cut the cabbage into small pieces and add them to the pan of hot water.


After a few minutes, filter the juice through a sieve and let it cool. Did you see it? The juice is pink!


With your brush, paint both sides of the filter paper with the red cabbage juice. Then let it dry.


In each glass, pour 1 cm of vinegar, soda, or baking soda solution. You can even ask your parents to run a little bleach. Next, insert your coffee filter so that the bottom of the filter is immersed in the liquid. Watch what happens!

Understand the experiment
Your coffee filter has a purple tint after being painted. However, when you put it in the vinegar, you observe that it turns pink! Better yet, it turns blue when you put it in the baking soda solution and even yellow when you try the bleach. Where do all these pretty colors come from? From the starting liquid? Not at all!

Why is red cabbage red?
Your cabbage contains a compound called anthocyanin. This molecule is responsible for its color. This compound can change color depending on whether it is in the presence of an acid like vinegar or a base like baking soda. That is why you observe these color variations when you introduce the coffee filter in the different cups.

A handy color scale for chemists
To classify compounds from the most acidic to the most basic, chemists have created a scale ranging from 0 to 14. They call this a pH scale. The closer the pH value is to 0, the stronger the acid. It is called “acid-strong”. The closer the value is to 14, the stronger your base is, like bleach. It is called a “strong base”. For a value of 7, it is called a neutral medium.
To test the pH of chemicals in the laboratory, chemists use colored indicators attached to the paper. These colored stripes are called pH paper.
The advantage of red cabbage is that you can see all the colors of the rainbow with anthocyanin. Therefore, it is effortless for you to know whether a product is basic, neutral, or acidic by observing the colors obtained on your scale. In your opinion, is your cola acidic, neutral or basic?
Did you know?
Nature changes color with pH
It is not only in laboratories that pH is measured with colored indicators. Nature itself tells us about changes in soil pH by changing color. For example, hydrangeas also contain anthocyanins. If the soil is “basic”, the flowers will remain blue. If the soil becomes acidic, the flower will turn pink. That’s when you know you need to add some heather to your plant.
Challenge
Once you have observed what happens when you dip your filter in the liquid of your choice, dry it. Then dip it back into another mixture of your choice. Can you see the color change?

Yeast’s inflating power
Here is a great and visual experience for you to discover the secret world of bacteria. They are very useful to us and we use them in many cooking recipes, for example. Watch how bacteria turn sugar into alcohol and carbon dioxide using a simple soda bottle and a balloon.
It’s a great way to identify which drinks have sugar and which doesn’t. […]
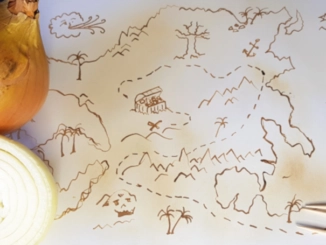
A treasure map with invisible ink
Invisible ink is very useful for drawing treasure maps or writing secret messages. Only those who know chemistry will find a way to reveal your message. Let’s use onion juice to prepare the invisible ink. […]

The volcano science experiment
Observing a chemical reaction in your kitchen is possible with the volcano science experiment. Build your volcano with sand. And then let’s move on to making lava. […]


